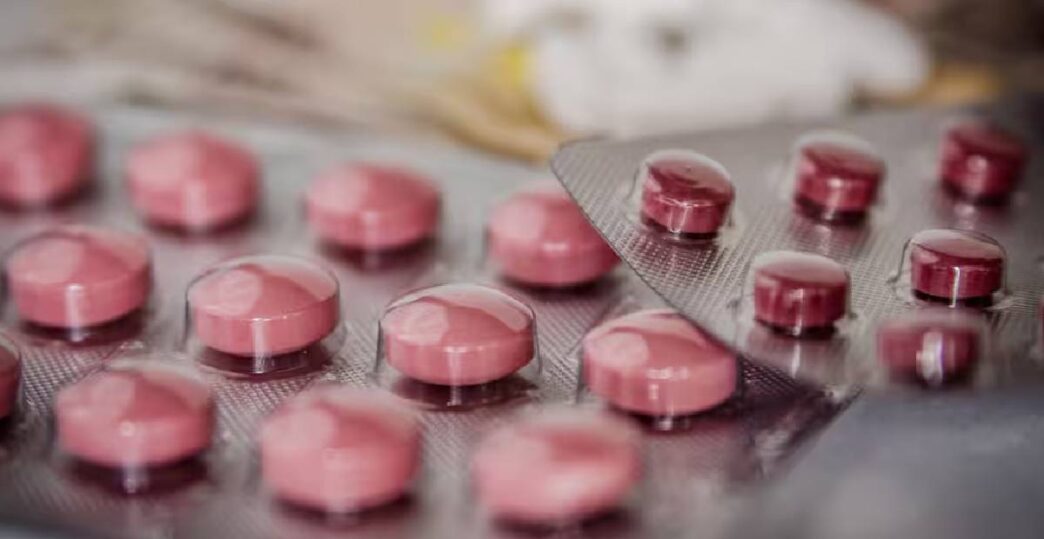
The Union Health Ministry has banned the manufacture, sale and distribution of all oral medicines that contain more than 100 milligrams of Nimesulide in an immediate-release form. The order has come into force with immediate effect.
The Central government issued the ban under Section 26A of the Drugs and Cosmetics Act, 1940, after discussing the matter with the Drugs Technical Advisory Board. The Health Ministry said that using Nimesulide in high oral doses could pose a risk to human health, and that safer alternatives are already available in the country.
Nimesulide is a non-steroidal anti-inflammatory drug (NSAID) commonly prescribed for pain and fever. Over the years, it has faced global scrutiny because of concerns related to possible liver damage and other serious side-effects. The latest decision by the Indian government is seen as part of a wider effort to strengthen medicine safety rules and remove products considered to be high-risk.
However, the ban applies only to doses above 100 mg meant for human use in immediate-release tablet or syrup form. Lower-dose formulations and other medicines used for similar purposes will continue to remain available in the market, the Ministry clarified.
Pharmaceutical companies that currently make or sell high-dose Nimesulide products have been asked to stop production immediately and recall existing stocks from the market. Industry analysts have said that the move may not cause a major financial impact on large drugmakers, because Nimesulide contributes only a small share to the total sales of pain and fever medicines. But smaller firms that rely more heavily on this product could face revenue challenges.
India has earlier used Section 26A to ban several fixed-dose combinations and high-risk medicines to protect public health. The latest move reinforces the government’s intention to keep a closer check on medicines considered unsafe.
Meanwhile, the government said that domestic capacity in the pharmaceutical sector continues to grow. Under the Promotion of Bulk Drug Parks scheme, investments worth more than ₹4,763 crore have already been made in the last three-and-a-half years until September 2025. This figure is higher than the originally committed amount of ₹4,329 crore over six years for greenfield projects.